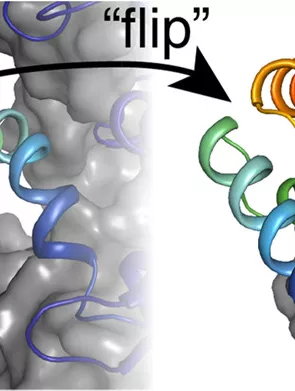
Chemistry's Edwin Ragwan has co-authored a publication with Professor Yan Kung titled "New Crystallographic Snapshots of Large Domain Movements in Bacterial 3-Hydroxy-3-methylglutaryl Coenzyme A Reductase," published in the journal Biochemistry.
Their paper is a look at a protein known as HMG-CoA reductase (HMGR). This protein is a pivotal enzyme used in all kingdoms of life where it is found in a metabolic pathway, responsible for producing tens of thousands of different types of natural product molecules called isoprenoids. Their study expanded the understanding of how HMGR works by revealing a never-before-seen position of a mobile region of the enzyme. Their work also confirmed important structural features that appear to dictate its physiological role. With this knowledge, Ragwan hopes that this paradigm will lead to a rational design of natural products that possess therapeutic value, such as novel antibiotics.